

Explain, including electron configurations in your answer. To the upper right corner of the periodic table will generally have the smallerĮxceptions to the trend above, particularly among the transition metals.Īnd fluorine from smallest to largest atomic radius. Will generally have the larger atomic radius, while an element located closer The lower effective nuclear charge is expected to have the larger atomicĬan draw the following conclusion regarding the trend on the periodic table forĪn element located closer to the lower left corner of the periodic table Than magnesium's 12 protons, chlorine will have a greater effective nuclearĬharge to draw chlorine's valence electrons closer to the nucleus and, thus,Ĭhlorine is expected to have the smaller atomic radius, while magnesium with Will generally have a greater effective nuclear charge to draw the valenceĮlectrons closer to the nucleus and, thus, decrease the atomic radius. Of n for the valence electrons, the atom with the greater number of protons The periodic table do not have a difference in the value of n for the valenceĪlkaline earth metal magnesium and the halogen chlorine in the third row both Sodium has the larger value of n than the valence 2s electron in lithium, we wouldĮxpect sodium to have the larger atomic radius. For example, let us compare the twoĪlkali metals lithium and sodium, which have the following electron Larger value of n will generally have the larger atomic radius. Same group of the periodic table, an atom having valence electrons with the You can effortlessly find every single detail about the elements from this single Interactive Periodic table.Periodic table are known as transition metals. Let me tell you how this Interactive Periodic Table will help you in your studies.ġ).
Atomic radius of calcium free#
Atomic number Elements Atomic Radius of Elements (pm) 1 Hydrogen (H) 120 pm 2 Helium (He) 140 pm 3 Lithium (Li) 182 pm 4 Beryllium (Be) 153 pm 5 Boron (B) 192 pm 6 Carbon (C) 170 pm 7 Nitrogen (N) 155 pm 8 Oxygen (O) 152 pm 9 Fluorine (F) 135 pm 10 Neon (Ne) 154 pm 11 Sodium (Na) 227 pm 12 Magnesium (Mg) 173 pm 13 Aluminum (Al) 184 pm 14 Silicon (Si) 210 pm 15 Phosphorus (P) 180 pm 16 Sulfur (S) 180 pm 17 Chlorine (Cl) 175 pm 18 Argon (Ar) 188 pm 19 Potassium (K) 275 pm 20 Calcium (Ca) 231 pm 21 Scandium (Sc) 211 pm 22 Titanium (Ti) 187 pm 23 Vanadium (V) 179 pm 24 Chromium (Cr) 189 pm 25 Manganese (Mn) 197 pm 26 Iron (Fe) 194 pm 27 Cobalt (Co) 192 pm 28 Nickel (Ni) 163 pm 29 Copper (Cu) 140 pm 30 Zinc (Zn) 139 pm 31 Gallium (Ga) 187 pm 32 Germanium (Ge) 211 pm 33 Arsenic (As) 185 pm 34 Selenium (Se) 190 pm 35 Bromine (Br) 183 pm 36 Krypton (Kr) 202 pm 37 Rubidium (Rb) 303 pm 38 Strontium (Sr) 249 pm 39 Yttrium (Y) 219 pm 40 Zirconium (Zr) 186 pm 41 Niobium (Nb) 207 pm 42 Molybdenum (Mo) 209 pm 43 Technetium (Tc) 209 pm 44 Ruthenium (Ru) 207 pm 45 Rhodium (Rh) 195 pm 46 Palladium (Pd) 202 pm 47 Silver (Ag) 172 pm 48 Cadmium (Cd) 158 pm 49 Indium (In) 193 pm 50 Tin (Sn) 217 pm 51 Antimony (Sb) 206 pm 52 Tellurium (Te) 206 pm 53 Iodine (I) 198 pm 54 Xenon (Xe) 216 pm 55 Caesium (Cs) 343 pm 56 Barium (Ba) 268 pm 57 Lanthanum 240 pm 58 Cerium (Ce) 235 pm 59 Praseodymium (Pr) 239 pm 60 Neodymium (Nd) 229 pm 61 Promethium (Pm) 236 pm 62 Samarium (Sm) 229 pm 63 Europium (Eu) 233 pm 64 Gadolinium (Gd) 237 pm 65 Terbium (Tb) 221 pm 66 Dysprosium (Dy) 229 pm 67 Holmium (Ho) 216 pm 68 Erbium (Er) 216 pm 69 Thulium (Tm) 227 pm 70 Ytterbium (Yb) 242 pm 71 Lutetium (Lu) 221 pm 72 Hafnium (Hf) 212 pm 73 Tantalum (Ta) 217 pm 74 Tungsten (W) 210 pm 75 Rhenium (Re) 217 pm 76 Osmium (Os) 216 pm 77 Iridium (Ir) 202 pm 78 Platinum (Pt) 209 pm 79 Gold (Au) 166 pm 80 Mercury (Hg) 209 pm 81 Thallium (Tl) 196 pm 82 Lead (Pb) 202 pm 83 Bismuth (Bi) 207 pm 84 Polonium (Po) 197 pm 85 Astatine (At) 202 pm 86 Radon (Rn) 220 pm 87 Francium (Fr) 348 pm 88 Radium (Ra) 283 pm 89 Actinium (Ac) 260 pm 90 Thorium (Th) 237 pm 91 Protactinium (Pa) 243 pm 92 Uranium (U) 240 pm 93 Neptunium (Np) 221 pm 94 Plutonium (Pu) 243 pm 95 Americium (Am) 244 pm 96 Curium (Cm) 245 pm 97 Berkelium (Bk) 244 pm 98 Californium (Cf) 245 pm 99 Einsteinium (Es) 245 pm 100 Fermium (Fm) – 101 Mendelevium (Md) – 102 Nobelium (No) – 103 Lawrencium (Lr) – 104 Rutherfordium (Rf) – 105 Dubnium (Db) – 106 Seaborgium (Sg) – 107 Bohrium (Bh) – 108 Hassium (Hs) – 109 Meitnerium (Mt) – 110 Darmstadtium (Ds) – 111 Roentgenium (Rg) – 112 Copernicium (Cn) – 113 Nihonium (Nh) – 114 Flerovium (Fl) – 115 Moscovium (Mc) – 116 Livermorium (Lv) – 117 Tennessine (Ts) – 118 Oganesson (Og) – Free Gift for you: Interactive Periodic Table (Note: Below mentioned radii are the van der Waals radius in picometer (pm) ).
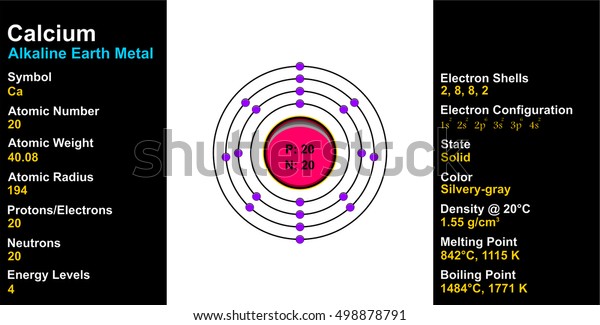
Atomic radius of all the elements are mentioned in the chart below.


 0 kommentar(er)
0 kommentar(er)
